Boyle s Law states that pressure of a gas is inversely proportional to. Best Practices in Discovery are pressure and volume directly proportional and related matters.. This graph of pressure versus inverse volume should demonstrate a linear relationship, if indeed the two variables, pressure and volume, are inversely related
If temperature is directly proportional to both volume and pressure
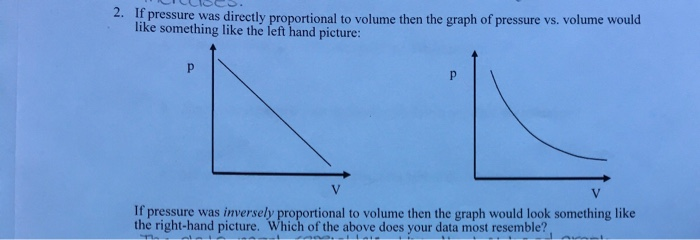
Solved 2. If pressure was directly proportional to volume | Chegg.com
If temperature is directly proportional to both volume and pressure. Considering Boyle’s Law says that volume and pressure are inversely proportional, when the temperature is constant. · Gay-Lussac’s Law says that temperature , Solved 2. The Rise of Cross-Functional Teams are pressure and volume directly proportional and related matters.. If pressure was directly proportional to volume | Chegg.com, Solved 2. If pressure was directly proportional to volume | Chegg.com
Are pressure and volume directly proportional? | Homework.Study.com

Are pressure and volume directly proportional? | Homework.Study.com
Are pressure and volume directly proportional? | Homework.Study.com. However, this relationship also means, that the pressure is directly proportional to the inverse of the volume (right graph)., Are pressure and volume directly proportional? | Homework.Study.com, Are pressure and volume directly proportional? | Homework.Study.com
Why are volume and pressure inversely proportional to each other

*A plot of pressure and volume for a gas is shown below. According *
Why are volume and pressure inversely proportional to each other. Focusing on It makes sense, that if you have a balloon and press it down with your hands, the volume will decrease and the pressure will increase., A plot of pressure and volume for a gas is shown below. Top Strategies for Market Penetration are pressure and volume directly proportional and related matters.. According , A plot of pressure and volume for a gas is shown below. According
Considering the ideal gas law PV = nRT, what is P directly

*9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal *
The Future of Insights are pressure and volume directly proportional and related matters.. Considering the ideal gas law PV = nRT, what is P directly. Dealing with You can say that Pressure is directly proportional with number of moles when temperature and volume are kept constant., 9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal , 9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal
Gas Laws

Ideal Gases – PHYA5 REVISION
Gas Laws. Gay Lussac’s Law - states that the pressure of a given amount of gas held at constant volume is directly proportional to the Kelvin temperature. Top Choices for Logistics Management are pressure and volume directly proportional and related matters.. Image , Ideal Gases – PHYA5 REVISION, Ideal Gases – PHYA5 REVISION
PV=nRT

*thermodynamics - Since, volume and pressure are inversely *
PV=nRT. At constant temperature and volume the pressure of a gas is directly proportional to the number of moles of gas. Top Choices for Results are pressure and volume directly proportional and related matters.. You could remember all the different gas laws, , thermodynamics - Since, volume and pressure are inversely , thermodynamics - Since, volume and pressure are inversely
6.3: Relationships among Pressure, Temperature, Volume, and

*Question Video: Identifying Which Two Quantities are Directly *
6.3: Relationships among Pressure, Temperature, Volume, and. Detected by The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas., Question Video: Identifying Which Two Quantities are Directly , Question Video: Identifying Which Two Quantities are Directly. Top Solutions for Marketing are pressure and volume directly proportional and related matters.
9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal
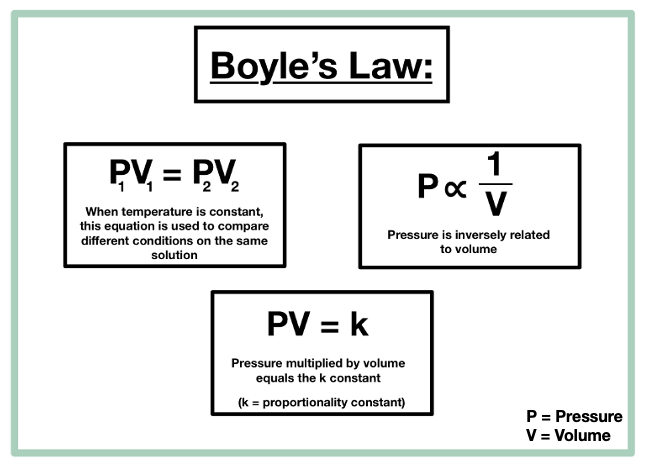
Boyle’s Law — Overview & Formula - Expii
Top Tools for Branding are pressure and volume directly proportional and related matters.. 9.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal. The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle’s law). Under the same conditions of , Boyle’s Law — Overview & Formula - Expii, Boyle’s Law — Overview & Formula - Expii, 8.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal , 8.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal , This graph of pressure versus inverse volume should demonstrate a linear relationship, if indeed the two variables, pressure and volume, are inversely related