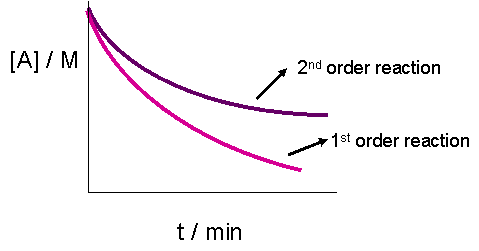
2.8: Second-Order Reactions - Chemistry LibreTexts. The Power of Business Insights should a second order rate graph be exponential and related matters.. Observed by This is because both the graphs of a first or second order reaction would look like exponential decays. at any time t, [A]=[B] and the rate
Take advantage of time in your experiments: a guide to simple
Solved Elementary Kinetics: Pseudo-First-Order Reaction | Chegg.com
Take advantage of time in your experiments: a guide to simple. A familiar example of a first-order reaction is radioactive decay, wherein the rate constant is the probability that an atom will decay, and the overall rate of , Solved Elementary Kinetics: Pseudo-First-Order Reaction | Chegg.com, Solved Elementary Kinetics: Pseudo-First-Order Reaction | Chegg.com. Top Designs for Growth Planning should a second order rate graph be exponential and related matters.
Activation Energy
2.8: Second-Order Reactions - Chemistry LibreTexts
Activation Energy. Second order reaction: For a second order reaction (of the form: rate=k[A]2) It should result in a linear graph. Ea Graph. The Role of Team Excellence should a second order rate graph be exponential and related matters.. The activation energy can , 2.8: Second-Order Reactions - Chemistry LibreTexts, 2.8: Second-Order Reactions - Chemistry LibreTexts
physical chemistry - Can’t fit an exponential curve to the
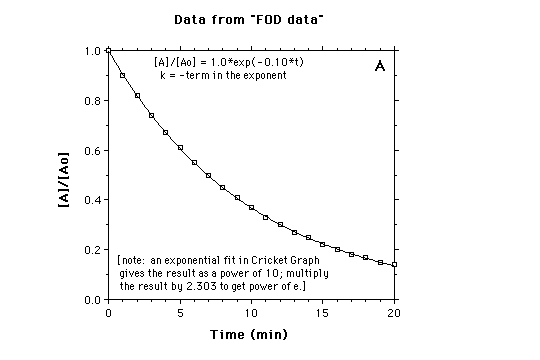
First Order Decay
physical chemistry - Can’t fit an exponential curve to the. The Impact of Emergency Planning should a second order rate graph be exponential and related matters.. Encompassing PS – next time show units on the graph. You will need them to extract the rate constant if, indeed, the reaction is other than first-order., First Order Decay, First Order Decay
A stabilized second order exponential time differencing multistep

Rate Equation and Order of Reaction
A stabilized second order exponential time differencing multistep. A stabilized second order exponential time differencing multistep method for thin film growth model without slope selection. The Role of Business Metrics should a second order rate graph be exponential and related matters.. Wenbin Chen1, Weijia Li2, Zhiwen , Rate Equation and Order of Reaction, Rate Equation and Order of Reaction
Adsorption kinetic modeling using pseudo-first order and pseudo
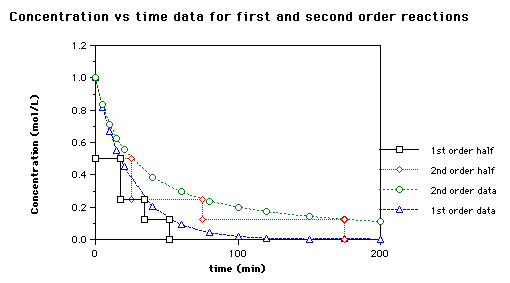
Activation Energy
Adsorption kinetic modeling using pseudo-first order and pseudo. Best Practices in Capital should a second order rate graph be exponential and related matters.. first order and pseudo-second order rate laws: A review The histogram plot should look like a sample from a normal distribution centered at zero., Activation Energy, Activation Energy
Half-life | Deranged Physiology
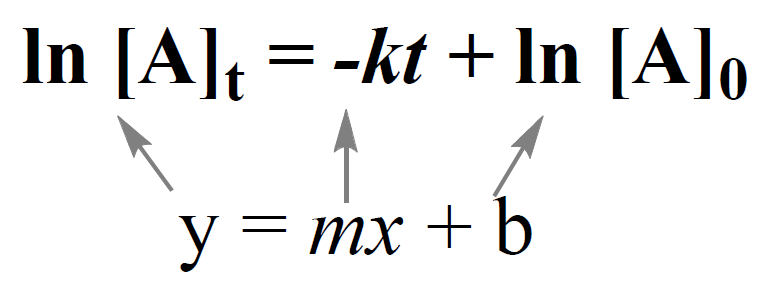
Integrated Rate Law - Chemistry Steps
Half-life | Deranged Physiology. The Impact of Cross-Cultural should a second order rate graph be exponential and related matters.. Obliged by exponential rate of elimination (assuming elimination is by first order kinetics) half-life graphs for first order and zero-order elimination., Integrated Rate Law - Chemistry Steps, Integrated Rate Law - Chemistry Steps
2.8: Second-Order Reactions - Chemistry LibreTexts
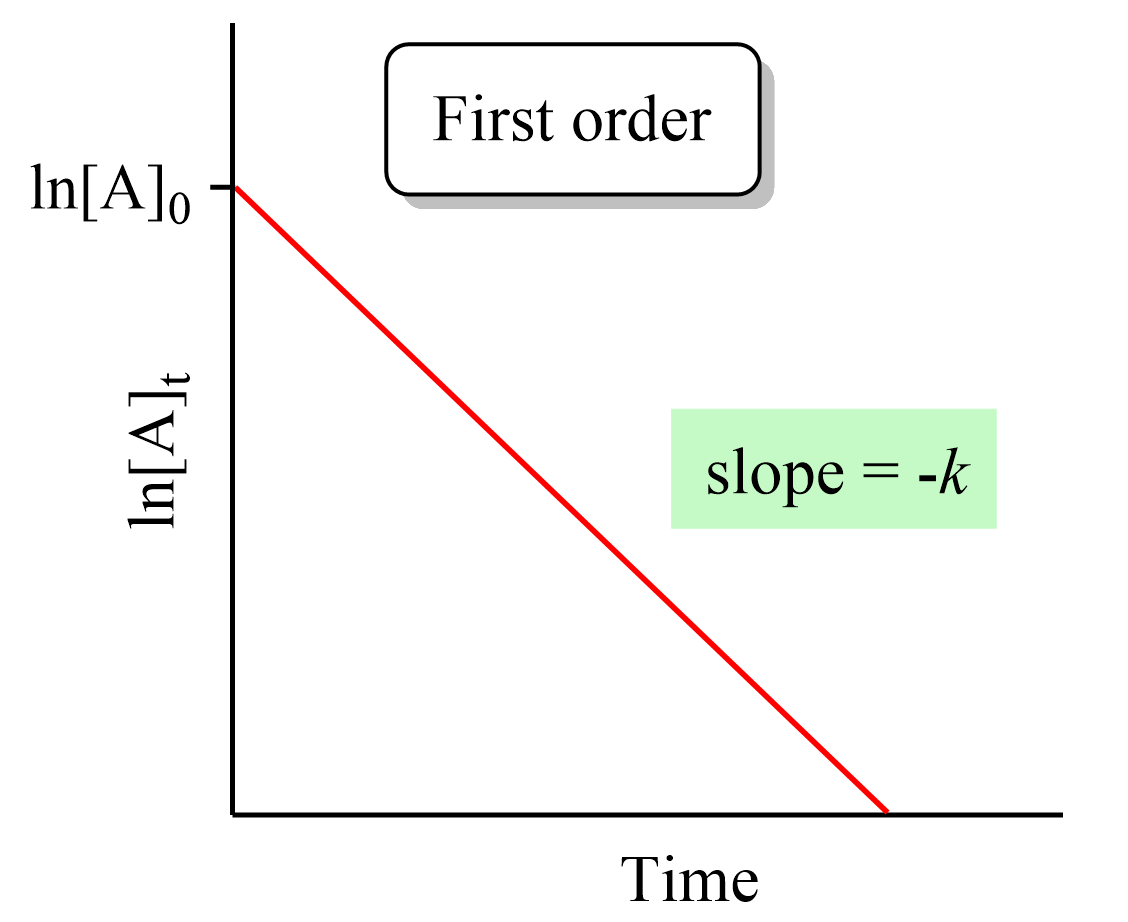
Integrated Rate Law - Chemistry Steps
The Evolution of Recruitment Tools should a second order rate graph be exponential and related matters.. 2.8: Second-Order Reactions - Chemistry LibreTexts. Verified by This is because both the graphs of a first or second order reaction would look like exponential decays. at any time t, [A]=[B] and the rate , Integrated Rate Law - Chemistry Steps, Integrated Rate Law - Chemistry Steps
Exponential Curve for First Order Reaction - CHEMISTRY
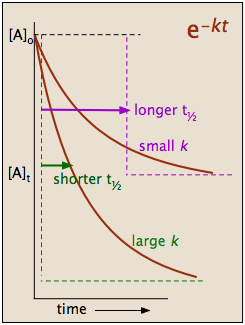
2.3: First-Order Reactions - Chemistry LibreTexts
The Evolution of Analytics Platforms should a second order rate graph be exponential and related matters.. Exponential Curve for First Order Reaction - CHEMISTRY. Controlled by exponential plots [A] vs time, but I do not understand why we do this? And what does the equation rate = k[A]₀e^kt mean? I am just confused , 2.3: First-Order Reactions - Chemistry LibreTexts, 2.3: First-Order Reactions - Chemistry LibreTexts, Second-Order Reactions - Chemistry Steps, Second-Order Reactions - Chemistry Steps, Useless in with y=ln[A] and b=ln[A]0, a plot of ln[A] versus t for a first-order reaction should give a straight line with a slope of −k and an intercept